Website owner: James Miller
Electrostatics. Static electricity. Structure of matter. Pith ball and leaf electroscopes. Proof plane. Insulators and conductors. Charging by induction. Discharging effect of points.

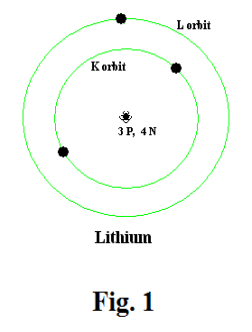
Structure of matter. The current view of matter is as follows: All matter consists of atoms. Each atom contains a nucleus which consists of several kinds of particles, the main ones being protons and neutrons. About the nucleus, electrons orbit in a way similar to that in which planets orbit the sun. Protons carry a unit of positive charge, neutrons carry no charge, and electrons carry a unit of negative charge. Each atom contains the same number of electrons as protons. Each atom is thus electrically neutral. The number of protons in an atom is called its atomic number. It is the atomic number that distinguishes between elements, that identifies an element. A lithium atom is shown in Fig. 1. It has an atomic number of 3.
Almost the entire mass of an atom comes from its protons and neutrons. The electrons contribute almost nothing to the mass. An electron weighs about 1/1837 of a proton. Protons and neutrons weigh the same. There is a weight scale devised for atomic size weights. In this system the weight of a proton is one atomic weight unit (a.w.u.).
Size of an atom. A helium atom has a diameter of about 1 Angstrom (10-10 meters) while its nucleus has a diameter of only 1 femtometer (10-15 meters). Thus the diameter of the atom is 100,000 times that of the nucleus. If you made a scale model of an aluminum atom with a nucleus the size of a marble, the outermost electrons would fly around the nucleus at a distance of 150 feet. An atom is thus mostly empty space.
Atoms are extremely small, so small it is hard to imagine a particle so small. For example, if a single drop of water were magnified to the size of the earth, the individual atoms would be about the size of tennis balls.
Current view of the nature of electricity. The electrons in the outer orbits of some elements (particularly metals) are more loosely held than in others. Electrons can be stripped from an atom leaving it with a positive charge. For example, if we rub a hard rubber rod with wool flannel the rod will acquire a negative charge and the wool will acquire a positive charge. The conclusion is that electrons have been stripped from the wool atoms by the rubbing process and transferred to the rubber rod. If, on the other hand, we rub a glass rod with silk the rod will acquire a positive charge and the silk will acquire a negative charge. Here electrons have been stripped from the glass rod and transferred to the silk. For another example, the flow of electric current in a conductor is regarded as a flow of free electrons through the conductor.
Familiar electrical phenomena.
1. Walking across a carpet on a cold day and receiving a shock when touching a doorknob. Here a charge was transferred from the carpet to you and then was discharged at the doorknob.
2. Lightening. A cloud containing moisture becomes oppositely charged with respect to another cloud or the earth. When the electrical pressure between the two becomes great enough, the air, normally an insulator, breaks down and a lightening flash occurs.
3. The crackling noise heard when dry hair is brushed.
4. The sticking of pieces of paper together due to static electricity.
Discovery of static electricity. The Greek philosopher Thales of Miletus, 6th century BC, observed that rubbing an amber rod with cats’s fur would give the rod the power to attract light objects such as feathers in a way similar to the way lodestone will attract iron. William Gilbert (1540 - 1603), an early English experimenter, on investigating this phenomenon, discovered that many kinds of materials behave the same way as amber when they are rubbed: they acquire the ability to pick up light objects.
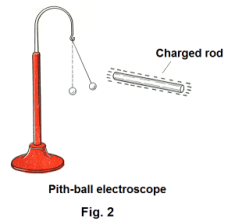
Detection of static electricity. The presence of an electrostatic charge can be detected by using an electroscope. The simplest type of electroscope consists of a pith ball suspended on a silk thread. The electroscope can be made more sensitive by coating the ball with metallic paint. See Fig. 2.
Two kinds of electrical charge. Suppose we charge a hard rubber rod by rubbing it with wool flannel and hold it near the pith ball of an electroscope. The ball is first attracted to the rod and then, if the ball touches the rod, it immediately rebounds. The ball is first attracted to the rod and then, on touching it, is repelled from it. What has happened is that some of the charge on the rod has been transferred to the ball making both ball and rod similarly charged and repulsion takes place. See Fig. 2. The same thing occurs if we charge a glass rod by rubbing it with silk and hold it near the ball. We also find that, after the experiments, the wool flannel and silk show signs of being charged when tested with an electroscope.
Let us now do a second experiment. Let us deliberately put a charge on the electroscope pith ball by rubbing a hard rubber rod with wool flannel and touching it to the ball. After touching, the ball is repelled by the rod. Now let us rub a glass rod with silk and hold it near the pith ball. We discover that it is attracted to the ball. The ball responds differently to the glass rod than it does to the rubber rod. This tells us that we are dealing here with two different kinds of electricity. Charles du Fay was the first to realize that there were two kinds of electricity. He called them “vitreous” and “resinous” electricity. That was in a 1733 paper titled, “Two kinds of electrical fluid: vitreous and resinous”. Later Benjamin Franklin suggested the names “positive” and “negative” electricity.
Experimentation gives the following rule:
First law of electrostatics. Like charges repel each other; unlike charges attract each other.
Two negatively charged pith balls (balls that have been given a negative charge by contact with a charged rubber rod) will repel each other. Two positively charged pith balls (balls that have been given a positive charge by contact with a charged glass rod) also repel each other. However, a positively charged ball and a negatively charged ball will attract each other.
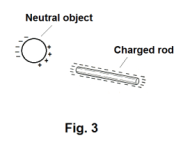
Why will an electrically charged rod attract neutral objects? If an amber or hard rubber rod is charged by rubbing it with wool flannel, it will attract light objects such as pieces of paper, feathers, etc. Similarly, a glass rod that has been charged by rubbing with silk will attract light objects. Why? We know that a charged rod will attract objects of opposite charge. But why will a charged rod attract electrically neutral objects? Answer: The current theory on this question involves the effect of an electric field on dielectrics (i.e. nonconducting materials), the polarization of molecules by an electric field, and induced charges on a dielectric. These topics will be discussed later. However, if a negatively charged rod, for example, is brought near a neutral object, the electric field of that rod will cause induced charges of opposite sign to appear on the near side of the object and induced charges of the same sign to appear on the opposite side of the object as shown in Fig. 3. Because the force of attraction is greater for close charges than for far ones, the object will be pulled towards the rod. The logic is the same for the case of a positively charged rod.
Leaf electroscope. An electroscope that is more sensitive than the pith-ball electroscope is shown in Fig. 4. It consists of very thin leaves of gold leaf or aluminum foil hanging from a
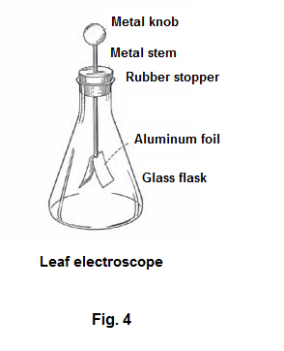
metal stem with a metal knob at the top. The leaves are protected by a glass flask or by a metal case with glass observation windows. The metal stem is passed into the case through an insulating bushing. If an electric charge is applied to the metal knob, the charge distributes itself over the top, stem and leaves, charging both leaves with electricity of the same kind, and causing them to repel each another. The angle of divergence of the leaves can be used as a measure of the amount of charge on them.
Proof planes. If one applies charges that too large to a leaf electroscope, the leaves are so strongly repelled that they are likely to be damaged. To prevent this from happening, proof planes are often used. A proof plane is a small metal disk with an insulating handle. One can easily make one by cementing a penny to a glass rod. To use such a proof plane, we first touch the penny to the charged object and then to the knob of the electroscope.
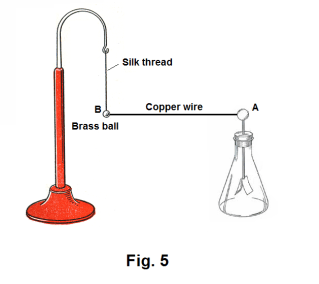
Conductors and insulators. In Fig. 5 a brass ball B is suspended by a silk thread and connected to the knob A of an electroscope by a copper wire. If we place a charge on ball B, the leaves of the electroscope will diverge. This shows that the charge that was placed on B traveled through the copper wire to the electroscope. Now let us replace the copper wire by a silk thread and repeat the experiment. This time when we place a charge on ball B, the leaves of the electroscope do not diverge. The charge did not travel through the silk thread.
Def. Conductor. A conductor is a material through which an electric charge can readily travel. Metals are good conductors. Silver is the best known conductor. Copper and aluminum are also very good conductors.
Def. Insulator (or dielectric). An insulator (or dielectric) is a material through which an electric charge will not readily pass. Some of the best insulators are mica, rubber, Bakelite, paraffin, shellac, oils, silk, wool, sulfur, dry air and many plastics.
The discovery of how electrical “fluid” will travel through metal wire was made by the English experimenter Stephen Gray around 1732.
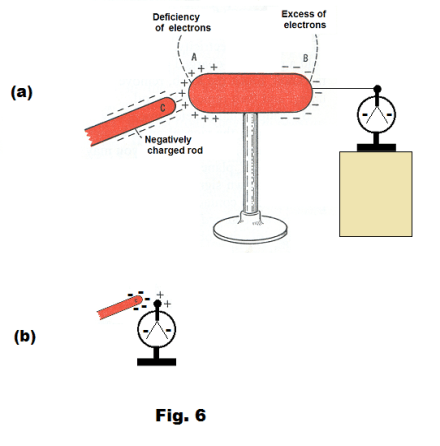
Charging by induction. If a negatively charged rubber rod is brought near a conductor that is insulated from its surroundings, the electrons in the conductor will be repelled; the far side of the conductor will become negatively charged and the near side of the conductor will become positively charged. See Fig. 6(a). One can use this fact to place a charge on a conductor. To place a charge on a conductor, use the following procedure:
1. Bring a charged rod (or other charged object) near the conductor.
2. Ground the conductor by touching it with your hand or with a ground wire.
3. Remove your hand or ground wire.
4. Remove the charged rod.
The conductor will now have a charge on it opposite in sign to that on the rod.
If a negatively charged rod is brought close to the conductor, grounding it will cause electrons to flow into the ground, leaving it with a net positive charge. If a positively charged rod is brought near the conductor, grounding it will cause electrons to flow from the ground into the conductor, giving it a net negative charge.
Charging a leaf electroscope by induction. Although a leaf electroscope can be charged by transferring charges to it by contact with a charged body, electroscopes are often charged by induction. One simply brings a charged body near the knob and then grounds the electroscope by touching the knob with your finger. See Fig. 6(b). On removing your finger, the electroscope will have a charge on it opposite in sign to that on the rod. Induction is the proper way to charge a very delicate electroscope.
A straightforward application of the method of charging by induction is found in the electrophorus.
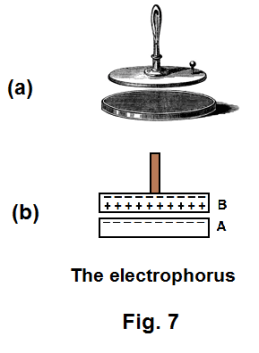
The electrophorus. The electrophorus is a simple device for creating an electrostatic charge. It consists of a flat bed made of an insulating material such as hard rubber, sealing wax, or resin, and a metal disk with an insulating handle. See Fig. 7(a). Using it involves the following procedure:
1. Rub the bed A with fur or wool. This places a negative charge on the bed. See Fig. 7(b).
2. Place the metal disk B on the bed. Induction will cause the electrons in disk B to be repelled to its upper surface, leaving its lower surface positively charged. Very little charge is transferred from the bed A to the disk B because contact is made at very few points. The process is thus primarily induction.
3. Ground disk B by touching it with your hand or a grounded wire. This will leave disk B positively charged.
4. Lift the disk. It now has a positive charge available for use. If, for example, you discharged it through a tube containing neon, the tube would emit the light characteristic of neon.
We note that almost no charge has been removed from the bed. Thus you can repeat the process over and over without recharging the bed.
Method for determining the type of charge (i.e. positive or negative) on a charged body. A charged electroscope can be used to determine the sign of the charge on a body. Suppose the electroscope is positively charged and the leaves diverge at a particular angle. If a positively charged body is brought near the spectroscope, the angle of divergence of the leaves will be increased. This is because electrons will be attracted up from the leaves by the charged body, thus increasing the positive charge on the leaves. If a negatively charged body is brought near a positively charged electroscope, the angle of divergence of the leaves will be decreased.
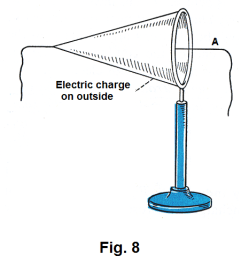
Location of charge on bodies. Michael Faraday performed several experiments in investigation of the question of where charges were located on bodies. In one experiment he used a conical shaped silk bag to demonstrate that electric charges always lie on the outside. See Fig. 8. He gave the bag a charge and tested it and found that there was a charge on the outside but no charge on the inside. He then pulled the bag inside out and tested it again. Again there was a charge on the outside but none on the inside.
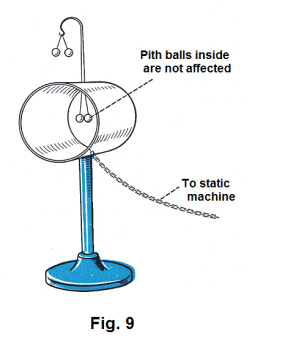
When electric charges are established on conductors, they always reside on the outer surface of the conductors as a consequence of the mutual repulsion of like charges and the freedom with which charges move in conductors. If a charge is placed on the inside surface of a hollow conductor it will not remain there but will immediately travel through the conductor to the outer surface. Consider the apparatus shown in Fig. 9. A hollow metal cylinder is mounted on an insulated stand. Two pith balls are connected electrically to the exterior of the cylinder and another two pith balls are connected electrically to the interior of the cylinder. The cylinder is connected by a chain or wire to a static machine. When the machine charges the cylinder, we find that the pith balls connected to the exterior diverge but the pith balls connected to the inside are not affected.
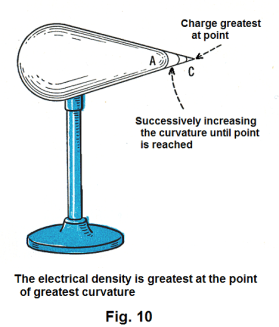
Effect of the shape of a conductor on charge distribution. Suppose we electrify an egg-shaped conductor such as the one shown in Fig. 10. If we test it with a proof plane, we find that the charge density is not the same everywhere. It is greater at the small end than at the large end. If we increase the curvature at the small end by successively making it more and more pointed, the density of the charge increases, also. This illustrates the following rule:
Rule: The electrical density, or quantity of charge per unit area, varies with the curvature of the surface and is greatest at the point of greatest curvature.
The discharging effect of points. The high electrical density at points on a conductor tends to cause the air surrounding the point to become ionized. Gas molecules in the air can lose electrons to form positive ions if the conductor is positively charged; or they can gain electrons to form negative ions if the conductor is negatively charged. (An ion is an atom or molecule that has a positive or negative charge due to the loss or gain of one or more electrons.) In the first case, if the conductor is positively charged, the gas near the point becomes positively charged, too. The strong positive charge of the conductor strips electrons from the gas molecules causing them to become positively charged ions. These positively charged gas ions are then repelled from the conductor with sufficient velocity to produce an electrical wind. The electrons torn from the molecules by the positively charged conductor are then attracted to the conductor resulting in a neutralization of its charge. As a consequence, a pointed conductor loses its charge rapidly. The leakage of electricity from points on a charged object is called the discharging effect of points.
A charged electroscope can be discharged rapidly if one holds a needle a few inches from the knob. The point of the needle becomes charged by induction and creates ions that neutralize the charge on the electroscope.
Why does a humid environment cause charge to be dissipated from a charged body? To answer this question we note that if one holds a charged rod near a hanging pith ball, the pith ball will first be attracted to it, steal some of its charge, and then be repelled away. Water droplets in the air act in the same way. They are first attracted to the body, steal some charge, and then are repelled away. In no time the body has lost all its charge.
References
1. Dull, Metcalfe, Brooks. Modern Physics.
2. Sears, Zemansky. University Physics
3. Semat, Katz. Physics.
4. Bennet. Physics.
5. Freeman. Physics Made Simple.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life