Website owner: James Miller
Transfer of heat. Conduction, convection, radiation. The ideal radiator. Blackbody.
The transfer of heat from one place to another takes place in three different ways: conduction, convection and radiation.
Conduction. Conduction is the flow of heat through a substance. Heat flows of its own accord through a body from a hot region to a cool region. The heat movement continues as long as a temperature difference exists and stops only when entire substance is of the same temperature. If one end of an iron rod is held in a fire, the other end soon becomes hot. The heat energy moves down the rod from the hot end to the cool end and stops when the entire rod is of the same temperature. Its behavior is similar to the behavior of water in flowing from a higher level to a lower level until all is at the same level — one possible reason why heat was once viewed as a fluid. According to the Kinetic Theory, heat is a form of energy, the kinetic energy of the random motion of molecules. In the warmer regions of a substance the molecules have more energy and vibrate more vigorously. When heat moves from a warmer region to a cooler region the molecular energy is passed along from molecule to molecule. Presumably, by collisions with adjacent molecules, energy is passed on, molecule by molecule.
Material |
Coefficient |
Silver |
100 |
Copper |
92 |
Aluminum |
50 |
Iron |
11 |
Glass |
0.20 |
Water |
0.12 |
Wood |
0.03 |
Air |
0.006 |
Wool |
0.010 |
Vacuum |
0 |
Heat conduction coefficients
Table 1
Heat moves more readily in some materials than in others. Metals are good conductors and heat moves readily through them. Thermal conduction appears to be related to electrical conduction in that good conductors of electricity are also good conductors of heat. Stone is a fairly good conductor. Wood, paper, cloth and air are poor conductors. In general, liquids are poor conductors and gases are even poorer conductors. Table 1 gives heat conduction coefficients for various materials. Silver is arbitrarily given the rating 100.
An experiment that shows how poorly water conducts heat is the following: Put some ice in the bottom of a test tube and a tuft of steel wool over it to hold it in place. Then fill the tube two thirds full with water and heat the upper part of the tube with a gas flame. The water in the upper part of the tube will boil for several minutes before enough heat is conducted down through the water to melt the ice.
Poor conductors of heat are often called heat insulators. Air, when trapped in small spaces, is an excellent insulator. Most of the warmth of woolens and fur is due to the trapped air between fibers. The best insulator of all is a good vacuum. A vacuum bottle is a double-walled glass flask with a vacuum between the walls.

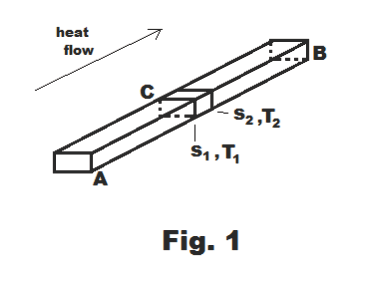
Rate of flow of heat in a conductor. Consider the metal bar AB shown in Fig.1. End A is maintained at a constant temperature TA and end B is maintained at a lower temperature TB creating a steady flow of heat from A to B. Let ΔQ/Δt be the quantity of heat flowing per unit time through the cross-section at point C and let s denote the distance along the bar from A to B with origin at point A. The temperature in the cross-section at s = s1 is T1 and the temperature in the cross-section at s = s2 is T2. The quantity
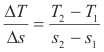
is the temperature gradient at point s = s1. We note that since T2 is less than T1, ΔT is negative and thus the gradient is negative. Then the quantity of heat flowing per unit time through the cross-section at point C is given by
![]()
where AC is the area of the cross-section at point C and k is a constant called the coefficient of thermal conductivity (i.e. heat conduction coefficient). The negative sign is introduced because the temperature gradient is negative (the quantity of heat flowing per unit time is proportional to T1 - T2 = - ΔT).
In the limit 1) above becomes
![]()
This equation is the general equation of heat conduction applying even in nonsteady heat flow conditions.
Convection. In the transfer of heat by convection, masses of matter in the form of gas or liquid transport the heat from one location to another i.e. convection currents of air, water, etc. carry heat from one point to another. Examples: the air currents of the earth’s atmosphere; ocean currents such as the Gulf Stream; circulating air, water or steam in home heating systems, etc. When air is heated by the sun or some other means it becomes less dense and rises, causing an upward air movement. Cooler air comes in under it, replacing it, and a convection current is set up. Unequal heating of the air at different places on the earth’s surface causes huge convection currents in the atmosphere. These convection currents are called winds. For example, sea breezes that blow from the sea towards the land during the day are caused by the fact that land heats up much more rapidly than water. As the land and air above it heat up on a warm day, the warmed air rises and the cooler air from the sea comes in under it forming a large convection current and causing a sea breeze. A sea breeze will blow from mid-morning to late evening.
Let us fill a large Pyrex glass container almost full with water and support it so that one side of it can be heated with a burner as shown in Fig. 2. We now drop several small crystals of potassium permanganate into the water. They will drop to the bottom and dissolve, forming a purple solution. As we heat the water on one side of the container, the water on that side expands, becomes less dense, and rises. As it rises, cooler, denser water comes in under it and replaces it. This water is in turn warmed and rises and a convection current is formed. Colored streams from the potassium permanganate show the movement of the water in the convection current.
The mathematical theory of heat convection is quite involved. There is no simple equation for convection as there is for conduction. This is due to the fact that when a surface at one temperature is in contact with a fluid at another temperature, the amount of heat transferred between surface and fluid depends on many things, such as:
1. Whether the surface is flat or curved
2. Whether the surface is horizontal or vertical
3. Whether the surface is smooth or rough
4. Whether the fluid in contact with the surface is a gas or a liquid
5. The density, viscosity, specific heat, and thermal conductivity of the fluid
6.Whether the velocity is small enough to give rise to laminar flow or large enough to cause turbulent flow
7. Whether evaporation, condensation or formation of scale takes place.
The procedure adopted in practice is to define a convection coefficient h by means of the equation
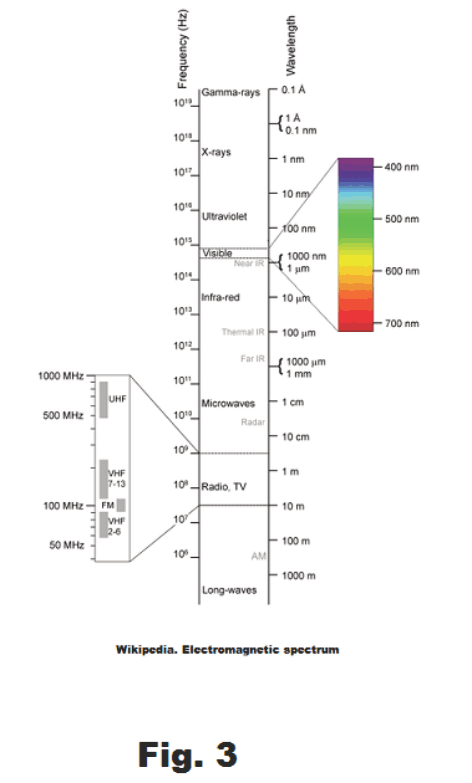
dQ/dt = hAΔT
where dQ/dt is the quantity of heat flowing per unit time, A is the area of the surface and ΔT is the temperature difference between the surface and the main body of the fluid. One then determines the value of h for a particular situation or piece of equipment. One finds in handbooks convection coefficients for various types of situations and equipment i.e. heat loss from vertical steampipes, horizontal steampipes, rough pipes, smooth pipes, etc.
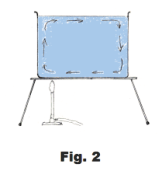
Radiation. The warmth that one feels by sitting in front of a hot stove or fireplace is not due to conduction or convection. It due to radiation, mostly radiation from the infrared portion of the electromagnetic spectrum. This band is located next to red band in the visible spectrum. It is of longer wavelength than the red and is invisible. See Fig. 3.
All bodies emit electromagnetic radiation. All bodies emit electromagnetic radiation, regardless of their temperature (i.e. all bodies with temperatures above 0o K emit radiation). Even blocks of ice emit radiation (including thermal radiation). However, as they are emitting radiation, they are also absorbing radiation from that being emitted by other substances in their environment in an interchange that may reach an equilibrium in which there is no net change if the other substances about them are of the same temperature as they. How much total electromagnetic radiation a body emits (of all wavelengths) varies directly with the fourth power of its Kelvin temperature according to Stefan’s law
R = eσT4
where
R = the rate of emission of radiant energy per unit area of the body
T = the Kelvin temperature
e = a quantity called the emissivity with a value between 0 and 1 depending on the surface
σ = a constant with a numerical value of 5.6699 × 10-5 in cgs units and 5.6699 × 10-8 in mks units
The thermal bands (bands associated with heat) are the visible and infrared bands. This thermal radiation is not heat itself. Rather the absorption of this radiation increases the thermal energy of the absorbing substance. The source of thermal radiation is presumably the internal vibrations of atoms and molecules of heat-producing bodies.
Electromagnetic radiation may be absorbed, reflected or transmitted. Of the electromagnetic radiation that impinges on a substance, how much is absorbed depends on both the wavelength of the radiation and the substance. With some substances the radiation of certain wavelengths may be absorbed while radiation of other wavelengths may be reflected or may just pass through with little being absorbed. When sunlight shines on a green leaf the wavelengths corresponding to the color green are reflected and the rest of the wavelengths are absorbed. When it shines on a yellow flower the wavelengths corresponding to the color yellow are reflected and the rest of the wavelengths are absorbed. Sunlight passes through ordinary glass with little absorption whereas the longer, invisible waves of infrared light do not, but are reflected. In a green house the visible rays of the sun pass easily through the roof and are absorbed by the soil. The soil emits rays of its own in the infrared range which are reflected by the glass and the green house acts like a heat trap. Thus some bodies may absorb much or most of the radiation impinging on them while others may reflect all or part of the radiation and others may just let the radiation pass through.
Thermal radiation passes through air readily, is absorbed by dark and rough surfaces and is reflected by shiny, smooth and light colored surfaces.
Good heat reflectors are poor absorbers. Polished metals are excellent heat reflectors. As a consequence they make poor heat absorbers. Rough surfaces absorb more heat than highly polished ones. The color of an object affects its absorbing power. Black surfaces absorb radiant energy while white ones reflect it. Thus white garments are more comfortable on a hot day than dark-colored ones. (A black object is black because it is absorbing all the visible wavelengths falling on it and reflecting none. A white object is white because it is reflecting all the visible wavelengths and absorbing none.)
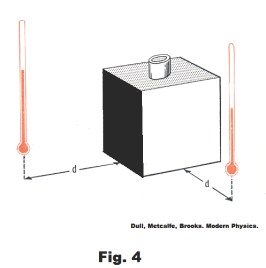
Good heat absorbers are good radiators and poor absorbers are poor radiators. In general, good absorbers of heat are also good heat radiators and poor absorbers are poor radiators. Consider the following experiment. In Fig. 4 is a cubical metal box, one side of which is polished metal and another side painted dull black. We fill the box with boiling water and place thermometers at equal distances from the two sides. The thermometer by the blackened side will show the higher reading. Thus this side must give off more radiant energy.
The ideal radiator. Because a good heat absorber is a good radiator, the best radiator will be that surface that is the best absorber. Any surface that absorbs all the radiant energy that strikes it will be the best possible radiator. Such a surface would reflect no radiant energy and consequently would appear black in color (provided its temperature is not so high as to make it luminous). Such a surface is called an ideal radiator, an ideal blackbody, or simply a blackbody. No real surface fulfills these conditions. Lampblack comes closest, reflecting only about 1% of the incident radiation. Blackbody conditions can be closely realized, however, by a small hole in the wall of a closed container. A hollow carbon box with a small hole in one side comes very close to an ideal blackbody. Radiation entering the hole will be reflected from wall to wall inside the box until it is absorbed. Very little radiation will be reflected out through the hole. If such a box is heated to a high temperature, the radiation coming out of the hole will be of greater intensity than the radiation from the same area of any other kind of surface at the same temperature. Stefan’s law states that the total radiation of all wavelengths coming from an ideal blackbody is proportional to the fourth power of its absolute temperature T i.e.
R = σT4
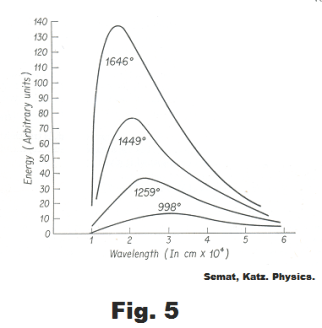
The emissivity e of a blackbody is 1. Fig. 5 shows the distribution of energy as a function of wavelength of a blackbody for various temperatures in oK. It is seen that as the temperature increases, the wavelength of maximum intensity decreases.
Heat transparency. A substance like dry air which is warmed little by the passage of thermal radiation is said to be transparent to it. Clouds and moist air are more opaque to thermal radiation. Thus, clouds absorb some of the sun’s thermal radiation. Some substances, such as alum, are transparent to visible light but opaque to thermal. On the other hand, iodine solution is opaque to visible light but transparent to thermal.
References
Dull, Metcalfe, Brooks. Modern Physics.
Sears, Zemansky. University Physics.
Schaum. College Physics.
Semat, Katz. Physics.
Freeman. Physics Made Simple.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life