Website owner: James Miller
Heat. Calorie, British thermal unit. Heat capacity. Specific heat. Method of mixtures. Changes of phase. Heat of fusion, vaporization. Sublimation. Evaporation. Liquefaction of gases. Critical temperature and pressure. PVT surface.
Units of heat. The three units most commonly used in measuring quantity of heat are calorie, kilocalorie, and British thermal unit defined as follows:
Calorie. The amount of heat needed to raise the temperature of one gram of water one Centigrade degree.
Abbrev. cal
Kilocalorie. The amount of heat needed to raise the temperature of one kilogram of water one Centigrade degree.
Abbrev. kcal or kg-cal
● 1 kcal = 1000 cal
British thermal unit. The amount of heat needed to raise the temperature of one pound of water one Fahrenheit degree.
Abbrev. Btu
● 1 Btu = 252 cal
Substance |
Specific Heat |
Water |
1.00 |
Alcohol |
0.65 |
Aluminum |
0.22 |
Glass |
0.20 |
Iron |
0.11 |
Copper, Brass |
0.09 |
Silver |
0.06 |
Lead |
0.03 |
Table 1
Specific heat. The number of calories needed to raise the temperature of one gram of a substance one Centigrade degree; or the number of British thermal units needed to raise the temperature of one pound of a substance one Fahrenheit degree. See Table 1.
● Specific heat is expressed as cal/g Co or Btu/lb Fo
● The specific heat is numerically the same in any system of units.
Example. The specific heat of iron is 0.107 cal/g Co. It is also 0.107 Btu/lb Fo.
In general, the specific heat of a substance varies with temperature. However, at ordinary temperatures, and over temperature intervals that are not too great, specific heats can be regarded as constant.
Heat capacity. The amount of heat needed to raise the temperature of a body one degree. It is measured in calories per degree centigrade (cal/Co) and in Btu per degree Fahrenheit (Btu/Fo)
Heat capacity = mass of body × specific heat
Note. The specific heat of a substance is the heat capacity of a unit mass of the substance. The specific heat of iron is the heat capacity of one gram of iron.
Phases of matter. In general, matter exists either as a solid, liquid or gas. These three states in which it may exist are called its phases. Provided they don’t decompose at high temperatures, all substances can exist in any of these three phases under the proper conditions of temperature and pressure. Water, for example, exists in the solid phase as ice, in the liquid phase as water, and in the gaseous phase as steam. Transitions from one phase to another are accompanied by the absorption or liberation of heat and usually by a change in volume.
Amount of heat Q gained (or lost) by a body in which there is no change of state. The amount of heat Q gained (or lost) by a body in which there is no change of state is given by
Q = mass × specific heat × temperature change
The Method of Mixtures. When two substances of unequal temperatures are brought into contact or mixed, the warmer one will lose heat and the cooler one will gain heat until both finally reach the same temperature. No heat energy is lost when substances of unequal temperatures are mixed. The heat lost by the hot substances will be equal to the heat received by the cooler substances. The number of calories lost by the warmer substances will be equal to the number of calories gained by the cooler substances. The formula that gives the calories (or Btu’s) gained or lost by a substance is
Q = mass × specific heat × temperature change
(provided there is no change of state).
____________________________________________________________________________
Problem 1. 100g of iron at 80o C are added to 107g of water at 25o C. What is the final temperature of the mixture?
Solution. Denote the final temperature by x. The specific heat of iron is 0.107. The number of calories lost by the iron is
100×0.107×(80 - x)
The number of calories gained by the water is
107×1.0×(x - 25)
Since the number of calories lost by the iron is equal to the number of calories gained by the water we have
100×0.107×(80 - x) = 107×1.0×(x - 25)
Solving this equation gives
x = 30
Problem 2. A brass cylinder weighing 201.9 g has been heated to a temperature of 100o C by suspending it in steam. It is put in a calorimeter containing 405 g of water at a temperature of 20o C. The calorimeter has a mass of 110 g and a specific heat of 0.09 cal/g Co. The final temperature of the water is 23.5o. What is the specific heat of the brass cylinder?
Solution. Let sc denote the specific heat of the brass cylinder.
The heat lost by the brass cylinder is :
201.9 × sc × (100 - 23.5)
The heat gained by the water is:
405 × 1.00 × (23.5 - 20) = 1417.5 cal
The heat gained by the calorimeter is:
110 × 0.09 × (23.5 - 20) = 34.7 cal
Since the heat lost by the brass cylinder is equal to the heat gained by the water and calorimeter, we have
201.9 × sc × (100 - 23.5) = 1417.5 + 34.7
sc = 0.094 cal/g Co
____________________________________________________________________________
The above method is a general one. In all problems involving heat exchange you identify the substances that lose heat and those that gain heat and remember that the heat lost by the warmer bodies is equal to the heat gained by the cooler bodies.
Fusion. The change of phase from a solid to a liquid.
Fusion is the scientific term for what is commonly called melting. The temperature at which fusion occurs is called the melting point. Pure substances generally have a definite melting point. Different solids have different melting points. Non-crystalline substances like glass and paraffin have no definite melting point. When heated they soften and can then be bent, molded, or welded.
Solidification. The change of phase from a liquid to a solid.
The temperature at which solidification occurs is called the freezing point.
For crystalline substances the melting point and freezing point are the same temperature.
Volume changes during solidification. Most substances contract when they solidify. There are a few exceptions to this rule, however. Water is the most important one. Water expands to a volume of about 1.1 times its original volume when it freezes. Bismuth and antimony are two metals that expand when they solidify.
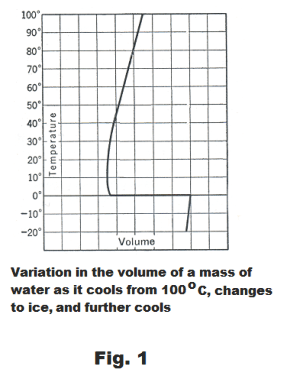
Volume change in water upon cooling. In Fig. 1 is a graph showing the change in volume of a mass of water as it cools from 100o C, changes to ice, and cools further. It contracts until it reaches a temperature of 4o C, then expands slightly as it cools to its freezing point, 0o C, where it then expands greatly in changing to ice. On further cooling below 0o, the ice contracts.
It is fortunate that water expands on freezing. If it contracted, the water in a lake would sink to

the bottom on freezing and the entire lake would freeze solid.
Effect of pressure on the freezing point of substances. An increase in pressure raises the freezing point of most substances. In substances such as water which expand on freezing, however, an increase in pressure lowers the freezing point.
Heat of fusion of a solid. The amount of heat required to melt 1 unit mass of the solid without changing its temperature.
Heat of vaporization of a liquid. The amount of heat required to vaporize 1 unit mass of the liquid without changing its temperature.
Heat of solidification of a liquid. The amount of heat given up in solidifying 1 unit mass of the liquid without changing its temperature.
Heat of condensation of a gas. The amount of heat given up in condensing 1 unit mass of the gas without changing its temperature.
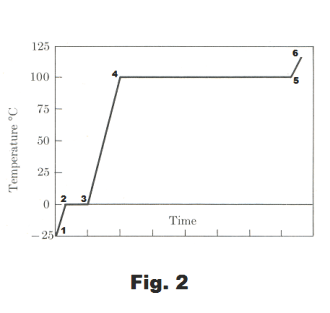
Changes of phase. Consider the following experiment: We take ice from a freezer where the temperature was -25o C, crush it quickly, place it in a container, and insert a thermometer in it. Imagine that the container is surrounded by a heating coil that supplies heat at a uniform rate and suppose no other heat reaches the ice. The temperature of the ice will be observed to increase steadily until it reaches 0o C as shown in the graph of Fig. 2 (segment from point 1 to 2). As soon as the temperature has reached 0o, some liquid water will start appearing in the container. The ice is now melting. The thermometer will show no increase in temperature, even though heat is still being supplied at the same rate as before, until all the ice is melted. (The ice and water must be kept thoroughly mixed, otherwise the part of the water closest to the heater will rise above 0o.). Once all the ice has melted (point 3 on the graph), the temperature will rise again at a uniform rate until it reaches 100o (point 4 on the graph). When this point is reached, bubbles of steam (gaseous water, water vapor) start to escape from the liquid surface. The water is now boiling. The temperature will remain constant, even though heat is still being continually supplied, until all the water has boiled away (point 4 to point 5 on the graph). If the water vapor had been trapped instead of being allowed to boil away, the heating process could be continued from point 5 to point 6. The gas would then be called “superheated steam”.
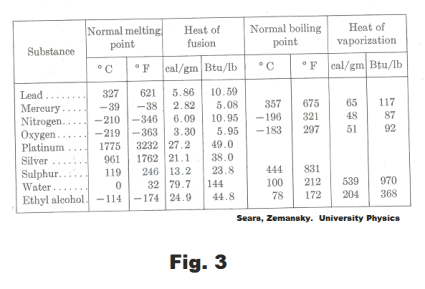
Between points 2 and 3, the ice is going through a change of phase from ice to water. In going through this change of phase the ice absorbs 79.7 calories (its heat of fusion) for each gram of ice. Between points 4 and 5, the water is going through a change of phase from water to steam. In going through this change of phase the water absorbs 539 calories (its heat of vaporization) for each gram of water. See Fig. 3.
We can now reverse the process. We can go from point 6 on the graph back to point 1, by removing heat. At point 5 the steam starts doing a change of phase, condensing, turning back to water. In doing this it gives up 539 calories (its heat of condensation) for each gram of gas. (We note that the heat of condensation is equal to the heat of vaporization.) When it reaches point 4 all the steam has been condensed into water. We continue removing heat until it reaches point 3. At point 3 it starts going through another change of phase, turning from water into ice. In doing this it gives up 79.7 calories (its heat of solidification) for each gram of water. Thus its heat of solidification is equal to its heat of fusion. When all water has been changed to ice we are at point 2. We can then continue removing heat until we get to point 1.
When a substance is going through a change of phase, two phases of the substance are together concurrently. Thus when water is going through a change of phase from water to ice, both water and ice are together concurrently as a mixture in equilibrium. The same is true when water changes to steam.
The above example used water but many substances follow the same type curve shown in Fig. 2. Some substances decompose before reaching a melting or boiling point. Others, such as glass or pitch, do not change state at a definite temperature but become gradually softer as their temperature is raised. Crystalline substances, such as ice or a metal, melt at definite temperatures.
Normal melting point. The temperature at which a crystalline solid melts when heat is supplied to it at atmospheric pressure.
Normal boiling point. The temperature at which a crystalline solid boils when heat is supplied to it at atmospheric pressure.
Sublimation. The evaporation of a solid.
Under the proper conditions of temperature and pressure, a substance can pass from the solid state to the gaseous state without passing through the liquid state. Camphor, iodine, naphthalene, and dry ice are some examples of substances which will evaporate at atmospheric pressure. Ice and snow are other examples.
Heat of sublimation. The amount of heat required to change 1 unit mass of a substance from solid to vapor at a given temperature.
Evaporation. Evaporation occurs when the surface molecules of a liquid leave the surface and dart off to become gas molecules.
Factors affecting rate of evaporation
1. Change in temperature. The rate of evaporation increases with an increase in temperature.
2. Size of surface area of the liquid. The larger the surface area of the liquid, the faster the liquid evaporates.
3. The nature of the liquid. Alcohol evaporates more rapidly than water. Some liquids such as olive oil don’t seem to evaporate at all.
4. The air pressure above the liquid. Evaporation becomes more rapid when the atmospheric pressure is reduced.
5. The amount of water vapor in the air above the liquid. The rate of evaporation is decreased when the amount of water vapor in the air above the liquid is increased.
6. Air movement above the liquid. The rate of evaporation increases with increased air movement over the liquid surface.
Liquefaction of gases. Michael Faraday (1791 - 1867) conducted an important series of
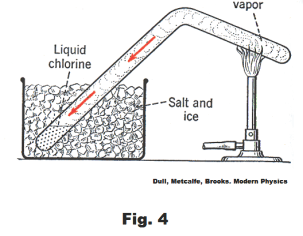
experiments on liquefying gases. In one experiment he filled a tube with chlorine gas using a thick-walled tube of the type shown in Fig. 4. He put one end of the tube in a freezing mixture of salt and ice and heated the other end. The gas in the heated end expanded and increased the pressure on the gas in the cold end and the combined effect of the pressure and the cooling liquified the chlorine. He used the same method to liquefy other gases, including ammonia and carbon dioxide, but was unable to liquefy oxygen, hydrogen and nitrogen in this way. We now know why he was unable to liquefy oxygen, hydrogen and nitrogen by this method. His freezing mixture was not cold enough to liquefy them no matter how much pressure is exerted on them. Oxygen must be cooled to -119o C, nitrogen to -147o C, and hydrogen to -240o C before they will liquefy at any pressure.
Def. Critical temperature. The temperature to which a gas must be cooled before it will liquefy.
Def. Critical pressure. The pressure needed to liquefy a gas at its critical temperature.
All known gases have now been liquefied by greatly compressing them, cooling them, and then allowing them to expand, a process that cools them further.
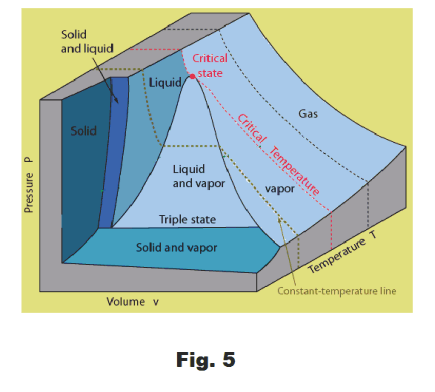
Pressure -Volume -Temperature (PVT) surfaces. Fig. 5 shows the Pressure - Volume - Temperature (PVT) surface of a typical substance. A PVT surface shows the particular phase in which a substance exists (solid, liquid, or gas) for any given set of values of the pressure, volume and temperature (P, V, T) for a unit mass of the substance.
That surface that corresponds to the collection of all possible (P, V, T) triples that can exist for a unit mass of a substance is called its PVT surface. A PVT surface can be viewed as an empirical equation of state of the substance.
The PVT surface thus shows those areas for which a substance exists as a gas, as a liquid, as a solid, etc.
References
Dull, Metcalfe, Brooks. Modern Physics.
Sears, Zemansky. University Physics.
Schaum. College Physics.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life