Website owner: James Miller
Colloids
Colloids. Colloid is a word that comes from the Greek word kolle, meaning glue. It was originally applied only to glue-like substances such as starch or gelatin. These substances when mixed with water pass through animal and vegetable membranes very slowly or not at all. This fact was discovered in 1861 by a Scottish chemist, Thomas Graham (1805-1896), who performed a series of experiments with such substances as starch, glue, salt, and sugar. In the experiments he enclosed water solutions of the materials in parchment bags and then suspended them in water. He noted that solutions of substances that crystalize well, such as sugar and salt, readily passed through the walls of the parchment bag and solutions of substances that didn’t crystalize didn’t. Since Graham’s time the word colloid has been broadened to include any dispersion of particles of very fine size that are larger than molecules.
Def. Colloid. A mixture in which very small particles of one substance are distributed evenly throughout another substance. The particles are generally larger than those in a solution, and smaller than those in a suspension, ranging in size from about one millionth of a millimeter to one hundred millionth of a millimeter.
Examples: butter, milk, smoke, fog, ink, paint
The particles may be any one of the three states of matter: solid, liquid, or gaseous. The dispersing agent in which they are suspended may also be solid, liquid, or gaseous.
Colloidal dispersions Examples
Solid in a solid Some alloys of metals, colored glass
Solid in a liquid Liquid glue, white of an egg, India ink
Solid in a gas A cloud of smoke in the air
Liquid in a solid Jelly, gelatin desserts
Liquid in a liquid Milk, mayonnaise, other emulsions
Liquid in a gas Fog, clouds in the sky
Gas in a solid Bread dough, marshmallow
Gas in a liquid Gas in ginger ale or soda water, whipped cream
Properties of colloids. Colloids have the following properties:
1. Non-settling. They are non-settling. Some larger colloidal particles may settle very slowly but finer particles remain suspended indefinitely.
2. Exhibit Brownian movement. They exhibit Brownian movement (i.e. the particles are in constant motion, following random, zigzag paths).
3. Exhibit Tyndall effect. They exhibit the Tyndall effect (i.e. the fine particles scatter light) causing a diffusion of light passing through them.
4. Enormous adsorptive power. They exhibit enormous adsorptive power due to the enormous surface area of the myriad of particles.
5. Electrical charge. The particles usually acquire an electrical charge, either positive or negative.

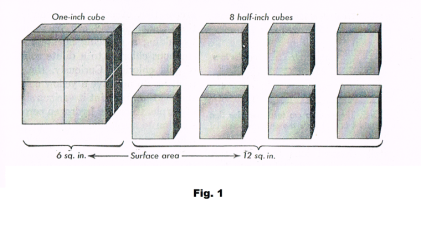
Large surface area of a colloidal suspension. Because of their small size, a given mass of colloidal particles has an astonishing total surface area. Suppose we divide a one-inch cube as shown in Fig. 1, producing 8 half-inch cubes. The surface area of the one-inch cube is 6 sq. in. The total surface area of the eight half-inch cubes is 12 sq. in. [ 8•6•(½)2 = 12 ]. Thus this operation has doubled the surface area. If we now apply the same operation to each of the eight half-inch cubes, we will double their total surface area to 24 sq. in. If we now repeatedly do this until we arrive at particles the size of colloidal particles we come up with an astonishing total surface area of over 200 acres! This gives colloids tremendous adsorptive power. An example is activated carbon which, in a gas mask, will adsorb many times its own weight in poisonous gases.
Electrical charge acquired by colloids. Examples of colloidal particles which acquire a positive charge include ferric hydroxide, aluminum hydroxide, albumen, and hemoglobin. Examples of colloidal particles which acquire a negative charge include clay, silver chloride, starch, oil emulsions, arsenic sulfide, activated charcoal, silicic acid, and metals.
Methods of precipitating colloids.
1. Heat. Heat causes the particles of some colloidal dispersions to coagulate. An example is dropping an egg into hot water to poach it. The heat coagulates the colloidal suspension of albumen, forming a white mass.
2. Adding an acid or base to neutralize the charge of the colloidal suspension. Latex, the natural fluid from the rubber tree, which is slightly alkaline, can be made to release the rubber by the addition of formic acid or acetic acid. This causes the rubber particles to coagulate. Another example is found in the manufacture of soap, where common salt is added to cause the colloidal soap particles to coagulate and collect on top of the suspension in which the soap in formed.
Methods of producing of colloidal suspensions. There are two general methods of producing colloids, called condensation and dispersion:
1. Condensation. Increase the size of extremely tiny particles up to colloidal size.
2. Dispersion. Break larger particles down to colloidal size.
Preparing colloidal suspensions by condensation - examples.
Example 1. If one adds hydrogen peroxide to a solution of hydrogen sulfide in water, colloidal particles of sulfur are dispersed in the water. That the particles are colloidal becomes apparent if we try to filter the mixture. The particles are so small that they pass right through the filter paper.
Example 2. We can produce colloidal ferric hydroxide by adding ferric chloride solution slowly to boiling water.
Methods of preparing colloidal suspensions by dispersion.
1. The electric arc
2. Grinding
3. Homogenizing
4. Shaking
The electric arc. One can produce an electric arc under water by momentarily touching two wires and then separating them just far enough so the arc is maintained. If the wires are of gold, colloidal particles of gold are torn loose from the wires, creating a colloidal suspension of gold. Such a colloidal suspension remains suspended indefinitely. Colloidal suspensions of other metals may be made in a similar way.
Grinding. Cement manufactures grind their product to colloidal size in large grinding mills.
Homogenizing. Homogenizers are colloid mills that usually have a grooved cone that rotates at very high speeds. The material to be homogenized is forced through a narrow slit that separates the rotating cone from a stationary housing. A shearing action breaks up the material into particles of colloidal size. Milk is homogenized to break up the fat globules into colloidal size.
Shaking. If we shake a few drops of oil in a jar of water, the oil will be broken up into colloidal particles dispersed in the water. In this case, the dispersion is not permanent, as the water will soon separate and come to the top.
Methods of stabilizing colloids.
Example 1. In manufacturing a graphite lubricant called aquadag, tannin is used to stabilize the suspension of graphite.
Example 2. When a pharmacist makes an emulsion he triturates the oil and the dispersing medium with gum arabic or gum tragacanth which makes the emulsion much more stable.
Example 3. Manufacturers of mechanical emulsions, toilet creams, and tooth pastes stabilize the colloidal dispersions in their products by grinding them in colloid mills.
References
1. Dull, Metcalfe, Brooks. Modern Chemistry.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life