Website owner: James Miller
Solutions. Solubility. Laws of ideal dilute solutions. Raoult’s law. Osmosis and osmotic pressure. Van’t Huff’s Law. Henry’s law.
Solvents. A solvent is a substance, usually a liquid, capable of dissolving other substances. Water has been called the universal solvent because of its remarkable ability to dissolve so many substances including solids, gases and other liquids. It shows at least some solvent action against nearly everything. Chemical actions usually take place readily in solutions and much of chemistry, both in the lab and in industry, is concerned with water solutions. Other common solvents include alcohol, gasoline, ether, turpentine and carbon tetrachloride. Some of these solvents dissolve certain substances that are almost completely insoluble in water. Solutions in which alcohol is the principal solvent are called tinctures or spirits. Usually tinctures are alcoholic solutions of nonvolatile substances and spirits are solutions of volatile substances.
Almost any liquid, solid, or gas may act as a solvent and dissolve other solids, liquids, or gases. For example, air can be viewed as a solution in which one gas is dissolved in another. Molten steel is a solvent which will dissolve carbon. After it solidifies, one solid is dissolved in another.
Def. Solution. A homogeneous mixture formed by dissolving one or more substances, whether solid, liquid, or gaseous, in another substance.
Solute and solvent. In a solution the dissolved substance is called the solute and the substance in which the solute is dissolved is called the solvent. In a solution consisting of the mixture of two liquids, the liquid present in the larger amount is generally regarded as the solvent.
Properties of solutions. Let us add two or three crystals of potassium permanganate to a liter of water and stir the mixture until the crystals have dissolved. This gives us a solution in which potassium permanganate is the solute and the water is the solvent. If we were to now try to filter out the potassium permanganate by passing the solution through some very fine filter paper we would not be able to do so. The particles of potassium permanganate are too tiny to be removed by the finest filter paper. Examination of the solution by the highest powered microscope would not reveal the particles. We conclude that they are very small. If we put the solution in a stoppered bottle so that the solvent doesn’t evaporate, the solute will not separate from the solvent.
Properties of solutions:
● a solution is a homogeneous mixture of two or more substances
● the particles of solute in a solution cannot be seen by the naked eye
● the mixture is stable and the solute will not separate or settle out
● the solute from a solution cannot be separated by filtration (or mechanically)
● a solution does not allow beams of light to scatter
We note that a solution is not a compound. This is evident because in a solution the proportions of solute and solvent can vary widely and in a compound constituents combine in a definite proportion.
Def. Miscible. Capable of mixing.
When two liquids are mutually soluble in each other, they are said to be miscible. Water and alcohol, for example, are miscible in all proportions. It doesn’t really matter which is called the solvent and which is called the solute. Glycerin and water are two other liquids that are miscible in any proportions.
Def. Immiscible. Not capable of mixing.
Oil and water are not soluble in each other. They are said to be immiscible.
Def. Emulsion. A liquid mixture in which a fatty or resinous substance is suspended in minute globules, as butter in milk.
When a small amount of oil is shaken vigorously with water, the oil becomes broken up into tiny particles that will remain in temporary suspension in the water for hours or even days. This is an emulsion.
Dilute, concentrated and saturated solutions. A solution containing only a small amount of solute is said to be dilute or weak. If we add more solute, the solution becomes stronger, more concentrated.
We weaken a solution by adding more solvent. We strengthen it, make it more concentrated, by adding more solute.

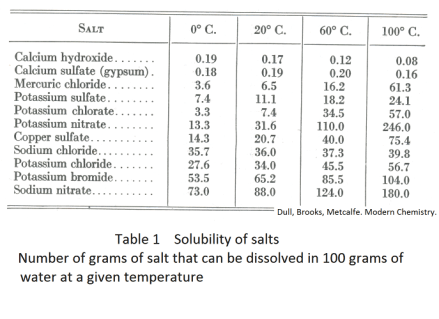
If we continue to add solute to a solution, the solution becomes more and more concentrated and the solute dissolves more and more slowly. The more concentrated the solution becomes, the slower it dissolves the solute. Finally a point is reached when the solution will dissolve no more solute. At this point the solution is said to be saturated. If we add more solute after this point, the extra solute will simply remain in the solvent undissolved. The amount of solute that a solvent will dissolve depends on temperature. For example, at 0o C, 13.3 g of potassium nitrate will dissolve in 100 ml of water. At 60o C, 110 g will dissolve.
Def. Solubility of a substance. The amount of the substance that will dissolve in a given quantity of solvent at a given temperature.
Solubility of solids. The solubility of a solid depends on three things:
1) the solid
2) the solvent
3) the temperature
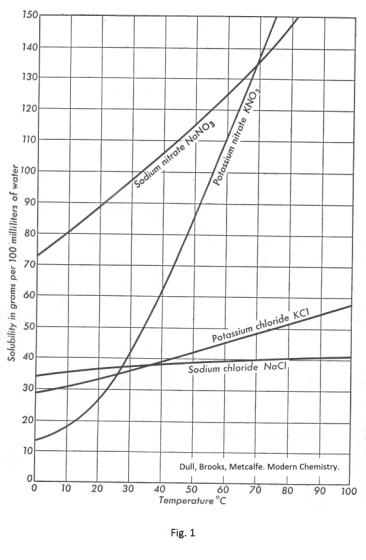
Table 1 gives the solubility of various salts in water at different temperatures. Fig. 1 shows graphs of solubility v.s. temperature for some salts. We see that the solubility of some salts (such as table salt, NaCl) change only moderately with temperature and the solubility of others (such as potassium nitrate) change dramatically.
The solubility of most solids increases with increasing temperature. There are a few exceptions — such as calcium hydroxide and calcium sulfate which are slightly more soluble in cold water than in hot water.
Ways of making a substance dissolve more quickly:
1) Powdering the solid. The dissolving action of the solvent occurs only at the surface of the solid. Powering the solid vastly increases the surface area. Powered solids will dissolve much more quickly than lumps of the same solid.
2) Stirring. Stirring increases the surface area by breaking up lumps and spreading out the solute, thus bringing more solvent into contact with undissolved solid
3) Heating the solvent. Increasing the temperature of the solvent not only increases the total amount that can be dissolved, but also increases the rate at which it dissolves.
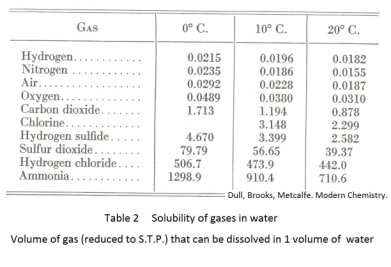
Solubility of gases. Gases are more soluble in cold solvents than in hot. Cold water, for example, will hold more oxygen than warm water. The solubility of a gas decreases as the temperature of the solvent is increased. Table 2 gives the solubility of various gases in water for various temperatures.
The solubility of a gas is affected not only by temperature but also by pressure. The solubility is increased by increasing the pressure of the gas above the solution. For example, carbonated beverages are made by forcing carbon dioxide into tanks of flavored water under a pressure of 5 to 10 atmospheres. When the pressure is reduced to one atmosphere, as when the beverage is drawn from a soda fountain, much of the gas will bubble off.
Solubility of gases. The solubility of a gas depends on four things:
1) the gas
2) the solvent
3) the temperature
4) the pressure
The laws of ideal dilute solutions. Just as gases in general tend to act in accordance with a group of simple laws, in a similar way dilute solutions tend to follow some simple laws. Solutions as a class have many properties that are determined by concentration alone without reference to the particular nature of the dissolved materials. “The laws of ideal dilute solutions” is an expression generally meant to include Raoult’s law, van’t Hoff’s law, and Henry’s law.
Vapor pressure of solutions. The vapor pressure of all solutions of non-volatile solutes in a solvent are less than that of the pure solvent. Let us form n different dilute solutions of equal molality by adding n different solutes A, B, C, ... to a particular solvent (because they are of equal molality each solution will have an equal number of solute molecules per kilogram of solvent) where the solutes are non-volatile, non-electrolytes. If we now measure the vapor pressures of the n solutions that we have prepared, we will find that the vapor pressures of all n solutions are depressed from that of the solvent by the same amount. We thus see that for the case of non-volatile, non-electrolyte solutes the amount of lowering of the vapor pressure depends only on the solvent and not on the solute.
Raoult’s law. Raoult’s law can be stated in two different forms:
1. In dilute solutions of non-volatile non-electrolytes the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent present i.e.
1) Vapor pressure of solvent above a solution
= vapor pressure of pure solvent × mole fraction of the solvent
2. In dilute solutions of non-volatile non-electrolytes, the depression of the solvent vapor pressure is equal to the vapor pressure of the pure solvent scaled by the mole fraction of the solute present i.e.
2) Depression of the solvent vapor pressure
= vapor pressure of pure solvent × mole fraction of the solute
__________________________________________________________________________
Problem. Compute the vapor pressure at 28o C of a solution containing 68 g of cane sugar, C12H22O11, in 1000 g of water.
The vapor pressure of water at 28o C is 28.35 mm.
Molecular weight of H2O = 18.02
Molecular weight of C12H22O11 = 342
Moles of C12H22O11 in 68 g = 68g / (342 g/mole) = 0.20 moles of C12H22O11
Moles of H2O in 1000 g = 1000g / (18.02 g/mole) = 55.49 moles of H2O
Total moles = 0.20 + 55.49 = 55.69 moles
Mole fraction of C12H22O11 = 0.20 / 55.69 = 0.0036
Mole fraction of H2O = 55.49 / 55.69 = 0.9964
First method.
Vapor pressure of solution = vapor pressure of pure solvent × mole fraction of the solvent
= 28.35 mm × 0.9964 = 28.25 mm
Second method.
Vapor pressure depression = vapor pressure of pure solvent × mole fraction of the solute
= 28.35 mm × 0.0036 = 0.10 mm
Vapor pressure of solution = (28.35 - 0.10) mm = 28.25 mm
__________________________________________________________________________
Def. Ideal solution. A solution in which the intermolecular attractive forces between the molecules of the solvent are the same as those between the molecules in the separate components and there are no volume changes or energy changes on mixing.
Pairs of chemically similar substances such as methanol (CH3OH) and ethanol (C2H5OH), or benzene (C6H6) and toluene (C7H8) form ideal solutions. Chemically dissimilar substances, such as C2H5OH and C6H6, form non-ideal solutions.
Raoult’s law for ideal solutions. In systems of liquids that mix with each other in all proportions to form ideal solutions, Raoult’s law in the form of 1) above applies to the partial pressure of each component separately:
3) partial pressure of any component above the solution
= vapor pressure of that pure component × mole fraction of that component
Freezing point of a solution. When most dilute solutions are cooled, pure solvent begins to crystallize before any solute crystallizes. The temperature at which the first crystals are in equilibrium with the solution is called the freezing point of the solution.
Relation between the freezing point of a solution and its molality. Because of the vapor pressure lowering, the freezing point of a solution is always less than the freezing point of the pure solvent. In dilute solutions, the lowering of the freezing point caused by a non-electrolyte solute is directly proportional to the molality of the solution (thus to the number of solute molecules per 1000 grams of solvent) and is given by
4) Lowering of freezing point = Kf × m
where m is the molality of the solution and Kf is the molal freezing point constant of the solvent. Kf represents the number of degrees by which the freezing point is lowered in a solution of 1 mole of any non-electrolyte dissolved in 1000 grams of the solvent. The molal freezing point constant for water is Kf = 1.86OC /m.
Example. If one mole of cane sugar (342 g of sugar) is dissolved in 1000 g of water, the solution will freeze at -1.86OC.
Relation between the boiling point of a solution and its molality. Because of the vapor pressure lowering, the boiling point of a solution is always higher than the boiling point of the pure solvent. For the case of dilute solutions of comparatively non-volatile solutes, the elevation of the boiling point is given by
5) Elevation of boiling point = Kb × m
where m is the molality of the solution and Kb is the molal boiling point constant of the solvent. Kb represents the number of degrees by which the boiling point is raised in a solution of 1 mole of any non-volatile non-electrolyte dissolved in 1000 grams of the solvent. The molal boiling point constant for water is Kb = 0.513OC /m.
Example. If one mole of cane sugar (342 g of sugar) is dissolved in 1000 g of water, the solution will boil at 100.513OC (at a pressure of one atmosphere).
Osmosis and osmotic pressure. Osmosis is the process in which a liquid passes through a membrane whose pores permit the passage of the solvent molecules but block the passage of the larger solute molecules.
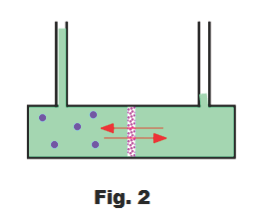
Fig. 2 shows a simple osmotic cell. A porous membrane separates the two compartments of the cell. Both compartments contain the solvent but the left compartment also contains a solute whose molecules are too large to pass through the membrane (the blue dots represent the solute molecules). Such a membrane is called a semi-permeable membrane. Many natural and artificial substances are capable of acting as semi-permeable membranes including animal bladders, the skins of fruits and vegetables, and the walls of most plant and animal cells.
If the cell is set up so that the fluid level is initially the same in both compartments, one will soon notice that the fluid rises in the left compartment and drops in the right compartment indicating that solvent is moving from the right side to the left side of the cell. This migration of the solvent is known as osmotic flow or osmosis.
What causes the solvent molecules to migrate from the right side to the left side? We note that the concentration of solvent molecules is lower in the left compartment than in the right compartment due to the presence of the solute molecules in the left compartment. The solvent molecules move from the right compartment to the left compartment due to the tendency of molecules to migrate from areas of high concentration to areas of low concentration in the same way that air molecules concentrated in the corner of a vacuous space will spread out to fill the space (the phenomenon is called diffusion). They are moving from the high concentration area of the right cell to the low concentration area of the left cell.
As the solvent migrates from the right compartment to the left compartment, the solution will rise in the extension tube above the left cell and the weight of the fluid in this extension tube will exert a downward pressure that will oppose the osmotic pressure pushing the solvent through the dividing membrane. When the weight of this column of fluid becomes equal to the osmotic pressure, the passage of solvent through the membrane will stop. This balance point at which the hydrostatic head of solution in the tube is equal to the driving force tending to equalize the concentrations on the two sides of the membrane is called the osmotic pressure of the solution.
The direct measurement of osmotic pressure is very difficult because of the enormous pressures that are involved. By one type device accurate measurements of 270 atmospheres have been made.
Van’t Huff’s Law. For dilute solutions the osmotic pressure, π, of solutions of non-electrolytes is given by
![]()
where
π = osmotic pressure in atmospheres
R = the ideal gas constant (R = 0.0821 l-atm mole-1 deg-1)
T= absolute Kelvin temperature
n = number of moles of solute present
V = volume of the solution (V/m is the molar concentration of the solute)
M = molarity of the solution
Deviations from the laws of ideal dilute solutions. The laws of ideal dilute solutions are valid only in dilute solutions of non-electrolytes. In the case of dilute solutions of electrolytes, each ion contributes independently to the effective molality of the solution; that is, each ion acts like a separate molecule in regard to the determination of vapor pressure, freezing point, boiling point, etc.. Because of the electrical interactions between ions, however, the effects are not as large as would be predicted on the basis of counting each ion as a molecule. For, example, a solution containing 0.100 gram-formula weight of KCl per kilogram of water freezes at -0.345oC whereas counting each ion as a molecule gives a predicted freezing point lowering of 2 × 0.1 × 1.86 = 0.372Co and thus a predicted freezing point of -0.372Co. Similarly, a solution containing 0.100 gram-formula weight of BaCl2 per kilogram of water freezes at -0.470oC whereas counting each ion as a molecule gives a predicted freezing point lowering of 3 × 0.1 × 1.86 = 0.558Co and thus a predicted freezing point of -0.558Co.
For any solution not too concentrated, the following hold:
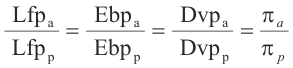
where
Lfpa = Lowering of freezing point (actual)
Lfpp = Lowering of freezing point (predicted)
Ebpa = Elevation of boiling point (actual)
Ebpp = Elevation of boiling point (predicted)
Dvpa = Depression of vapor pressure (actual)
Dvpp = Depression of vapor pressure (predicted)
πa = osmotic pressure (actual)
πp = osmotic pressure (predicted)
Henry’s law. At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Stated differently: The solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid:
References
Dull, Brooks, Metcalfe. Modern Chemistry.
Schaum, Beckmann, Rosenberg. College Chemistry.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life