Website owner: James Miller
Chemical bonding. Valence. Oxidation number. Determining chemical formulas. Naming of compounds. Balancing chemical equations.
Formation of compounds. In forming a compound, two or more atoms of different elements combine together to form a molecule. An atom of one element may combine with one or more atoms of certain other elements to form a compound, but only certain other elements, not any other elements. For example, atoms of iron will combine with atoms of oxygen to form an iron oxide. But atoms of iron will not combine with atoms of copper, or of lead, or of zinc, or atoms of many other elements. What is it that determines what elements will combine with each other to form compounds and what elements won’t combine with each other? What is it that holds the various atoms in a molecule together to create a distinct unit (the molecule) with its own distinct properties? What are the forces that hold the atoms of a molecule together and where do they come from?
Chemical bonding. The mechanism by which two elements (or an element and radical) unite to form a compound is generally considered to be the following: the electrons in the outer shell of one atom are either transferred to another atom or shared with another atom so as to make the outer shells of both atoms as complete as possible. Thus in the case of the compound H2O two hydrogen atoms each share their single electron with an oxygen atom (whose outer shell contains 6 electrons). By this sharing, the outer shell of the oxygen atom gains two electrons and thus becomes completed with 8 electrons, providing chemical stability.
Valence (or Oxidation number). When the atoms of two different elements combine, each atom either gains or loses electrons. The valence of an element is the net charge on an atom as given by the algebraic sum of the positive (proton) charges and the negative (electron) charges. When an atom is in its pure state its valence is 0 since it has the same number of electrons as protons. When an element is in a compound its valence will be either positive or negative depending on the number of electrons that it lost or gained in forming the chemical bond.
Syn. Valence number, Oxidation number, Oxidation state
Two categories of elements. The mechanism that determines how atoms combine to form molecules thus involves interactions involving the outer shell electrons of the atoms. We now consider two categories of elements:
1. Category A elements. Elements with one or two electrons in their outer orbit (or shell). This group includes the most of the metals. Copper, for example, has one electron in its outer orbit. Iron has two. Elements with one or two electrons in their outer orbits tend to have

them stolen away from them by other elements when forming a molecule. If they have their outer orbit electrons stolen away from them, they are said to be oxidized and their valence increases. (the term “oxidize” deriving from the fact that the element oxygen often steals electrons in this way). If copper has its outer orbit electron stolen,
Cu →Cu+ + e-
The valence of Cu is 0 and of Cu+ is +1. The valence corresponds to the net charge on the Cu+ ion (the Cu+ ion consists of the Cu atom minus an electron — it has n protons and n-1 electrons).
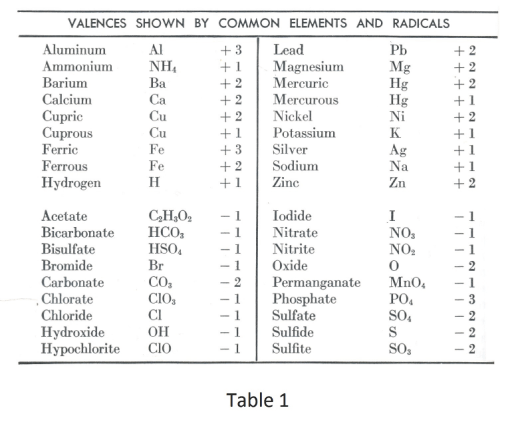
The valences of Category A elements are all positive when they are in compounds. Category A elements correspond to those elements shown in the upper half of Table 1.
2. Category B elements. Elements and radicals whose outer orbit lacks one or two electrons. This group includes oxygen and other nonmetals and many radicals. Elements whose outer orbit lacks one or two electrons tend to steal electrons from other elements in order to complete their outer orbit. Oxygen unites with many metals, and in doing so, steals their outer orbit electrons. If an element steals electrons from another element in the process of forming a molecule, it is said to oxidize the element and to be itself reduced. Its valence drops (becomes more negative due to increased electrons). For the case of oxygen,
O + 2e- → O-2
The valence of O is 0 and of O-2 is -2.
The valences of Category B elements are all negative when they are in compounds. Category B elements correspond to those elements shown in the lower half of Table 1.
In general, Category A elements tend to combine with Category B elements with the Category B elements utilizing the outer orbit electrons of the Category A elements – one element donates electrons and the other receives them. A Category A element bonds together with a Category B element to the benefit of both (creating a more stable state for both).
Chemical formulas. The chemical formula for a compound is an abbreviated way of indicating what a molecule of the compound consists of. The chemical formula for water is H2O. The formula tells us that a molecule of water consists of two atoms of hydrogen and one atom of oxygen. H is the symbol for hydrogen, O is the symbol for oxygen, and the subscript on the H gives the number of atoms of hydrogen in the molecule. The chemical formula for sulfuric acid is H2SO4. This formula tells us that a molecule of sulfuric acid consists of two atoms of hydrogen, one atom of sulfur and four atoms of oxygen. The symbol for sulfur is S.
Special note for formulas containing radicals. In chemical formulas containing multiples of a radical, the radical is regarded as a single unit and enclosed in parenthesis.
Examples. The formula for magnesium hydroxide is Mg(OH)2 and the formula for aluminum hydroxide is Al(OH)3. However the formula for sodium hydroxide is written NaOH. (If the radical occurs only once it is not put in parenthesis). The formula for ammonium sulfate is (NH4)2SO4 and the formula for aluminum sulfate is Al2(SO4)3.
Determining the chemical formulas of compounds from a table of valences. In writing the formula of a compound the total valence from the first, or positive part of the compound, must be equal but opposite in sign to the total valence of the second, or negative part of the compound. The total valence of an element in a compound is found by multiplying the valence of the element by the number of atoms of this element in the compound.
The sum of the total valences of the positive part of the compound and the negative part of the compound add to zero.
Example 1. In the compound H2O there are two atoms of hydrogen, each atom of hydrogen has a valence of +1, and so the total valence of the positive (hydrogen) part of the compound is +2. Oxygen has a valence of -2, there is only one atom of oxygen, and so the total valence of the negative (oxygen) part of the compound is -2. We thus see that the total valence of the positive part of the compound is equal but opposite in sign to the total valence of the negative part and the two add to zero.
Example 2. Consider the compound AlBr3 (aluminum bromide). Aluminum has a valence of +3 and bromine has a valence of -1. The total valence of the positive part is +3 and the total valence of the negative part is 3×(-1) = -3.
Example 3. Consider sulfuric acid, H2SO4. The positive part is H2 with a total valence of 2×(+1) = +2. The negative part of the compound is SO4 which is a radical viewed a single unit in figuring valences. It has a valence of -2. Thus the total positive and negative valences add to zero.
It should be noted that forming chemical formulas for compounds by the method described above utilizing the valence concept works for most compounds but not for all. There are exceptions. For example it would be difficult to explain the formulas for the well known compounds H2O2, C2H2, CaC2, Fe3O4, C2H4, or CO using the above assumptions on valence.
Naming of compounds. For most compounds the name consists of the name of the first (positive) part of the compound followed by the name of the second (negative) part of the compound. See Table1 for the names of the positive and negative parts of the compound. The name of the second (negative) part of the compound consists of a root plus a suffix such as -ide or -ate. Also, in some cases the root is preceded by a prefix such as “mon” or “di”. For example, in carbon dioxide (CO2) carbon is the name of the positive part of the compound, ox (for oxygen) is the root of the second (negative) part, “di” is the prefix, and “ide” is the suffix.
Examples.
NaCl sodium chloride
PbS lead sulfide
P2O5 phosphorous pentoxide
Mg(OH)2 magnesium hydroxide
Chemical equations. We now consider the process of writing a chemical equation. Let us reduce the process to a series of steps.
Step 1. Write out what occurs as a “word equation”.
Example. Water → hydrogen + oxygen (decomposition of water by electrolysis)
Step 2. Replace the word equation with one using the correct chemical formulas.
Example. H2O → H2↑ + O2↑
This equation is called a skeleton equation. On the left side of the equation, we write the symbols and formulas for all the reactants (i.e all substances entering the reaction). On the right side of the equation, we write the symbols and formulas for all the products that are formed as a result of the chemical reaction. An arrow separates the left and right sides of the equation. The arrow is read as “yields”. Gases are indicated by an up arrow.
What substances react with each other and what products are formed is information obtained from chemical experiment. Chemists over time have learned what substances react with each other and what the products are. One acquires this knowledge through reading and experience.
In writing the chemical formulas for various substances one must be aware that the gases hydrogen (H2), oxygen (O2), nitrogen (N2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2) are diatomic. This means that in the gaseous state they present themselves as two atoms bound together in a single molecule. In the equation they need to be written as H2, O2, N2, etc.
Step 3. Balance the equation. There must be the same number of atoms of each kind on the right side of the equation as on the left. If there are four oxygen atoms on the left side of the equation there must also appear four on the right side. The Law of the Conversation of Mass tells us that no atoms are gained or lost in a chemical reaction. Balancing the equation consists of determining coefficients for the different molecules on the left and right sides so as to satisfy the condition that there are the same number of atoms of each kind on the right side of the equation as on the left.
Example 1. Let us balance the skeleton equation
H2O → H2↑ + O2↑
We note that on the left side of the equation there is a single oxygen atom and on the right side there are two. We know there must be the same number of oxygen atoms on both sides so we start out by placing a 2 in front of the H2O to give
2 H2O → H2↑ + O2↑
This gives the same number of oxygen atoms on both sides but now there are four hydrogen atoms on the left side and only two on the right. So we try placing a 2 in front of the H2 to give
2 H2O → 2 H2↑ + O2↑
We see that there are now four H atoms on each side of the equation and two O atoms on each side so the equation is now “balanced”.
We balance an equation by trial and error. Complicated equations can be difficult to balance.
Example 2. Balance the skeleton equation
KClO3 → KCl + O2↑
There are three O atoms on the left and two on the right. We try the following to give the same number on both sides
2 KClO3 → KCl + 3 O2↑
This gives the same number of oxygen atoms on both sides but the number of K and Cl atoms are now different on the two sides. We place a 2 in front of the KCl:
2 KClO3 → 2 KCl + 3 O2↑
The equation is now balanced.
References
Dull, Brooks, Metcalfe. Modern Chemistry.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life