Website owner: James Miller
Acids, bases, salts. Normal solutions. Titration.
Acids. Acids are an important group of chemical compounds. Many are found in nature.
Examples of acids
● acetic acid (in vinegar)
● ascorbic acid (vitamin C)
● citric acid (in citrus fruit)
● tartaric acid (in grapes)
● tannic acid (in tea)
● lactic acid (in milk)
● sulfuric acid (H2SO4), nitric acid (HNO3), and hydrochloric acid (HCl) — acids widely used in industry in producing metals, plastics, explosives, textiles, and dyes
Def. Acid. A compound containing hydrogen which, in water solution, forms no positive ions other than hydrogen, H+, ions.
Note that the H+ ion is a proton. Thus an acid can be viewed as a proton provider.
Def. Base. A compound containing the hydroxyl group which, when dissolved in water, forms no negative ions other than hydroxyl, OH-, ions.
Note. A more general definition of a base is that it is any substance that can accept H+ ions i.e. protons.
Examples of bases
● ammonium hydroxide, NH4OH (household ammonia)
● sodium hydroxide, NaOH (lye)
● calcium hydroxide, Ca(OH)2 (present in limewater)
● magnesium hydroxide, Mg(OH)2 (milk of magnesia)
Def. Neutralization. The union of hydrogen ions of an acid with hydroxyl ions of a base to form water.
Salts. The word “salt” has a specialized meaning in chemistry. Salts are the compounds other than water that are produced when an acid neutralizes a base. A salt can be defined as follows:
Def. Salt. A compound whose water solution contains a positive ion other than the hydrogen ion and a negative ion other than the hydroxyl ion.
Examples of salts
● sodium chloride, NaCl (table salt)
● calcium carbonate, CaCO3
● barium chloride, BaCl2
● sodium sulfate, Na2SO4
Def. Indicator. A substance used to show, by means of a change in color, whether a solution is an acid or a base.
Properties of acids
1. All acids contain ionizable hydrogen and a nonmetallic ion or radical.
Examples.
1) Hydrochloric acid (HCl) in water solution yields
HCl → H+ + Cl-
2) Nitric acid (HNO3) in water solution yields
HNO3 → H+ + NO3-
3) Sulfuric acid (H2SO4) ionizes in two steps, depending on the amount of its dilution:
H2SO4 → H+ + HSO4-
HSO4- → H+ + SO4=
The first step represents the ionization in a rather concentrated solution. In a dilute solution both reactions occur. In a concentrated solution acid salts containing the HSO4- may be produced whereas in a dilute solution regular salts containing the SO4= radical are produced.
2. All acids have a sour taste.
Examples. Lemons, limes and grapefruit are sour. Vinegar is sour.
3. Acids cause some substances to change color, allowing them to be used as acid indicators.
Example. An acid will turn blue litmus paper red.
4. Acids neutralize bases. If we add an acid to a base in the proper proportions each will destroy the properties of the other.
5. Acids act on some metals, setting the hydrogen free and producing a salt.
Example. Zn + H2SO4 → ZnSO4 + H2↑
6. Acids react with the oxides of metals forming salts and water.
Example. CuO + H2SO4 → CuSO4 + H2O
7. Acids act on carbonates liberating carbon dioxide and producing a salt and water.
Example. CaCO3 + 2HCl →CaCl2 + CO2↑ + H2O
Sulfuric acid (H2SO4). A dense, oily liquid with a high boiling point. Concentrated sulfuric acid contains about 95% sulfuric acid and 5% water. Ordinary dilute sulfuric acid is made by pouring 1 part of concentrated sulfuric acid into 6 parts of water. The specific gravity of concentrated sulfuric acid is 1.84.
Nitric acid (HNO3). 100% nitric acid is a volatile liquid that is too unstable to be put on the market. Commercial concentrated nitric acid is fairly stable and contains 68% nitric acid dissolved in water. Ordinary dilute nitric acid is usually made by adding 1 part of nitric acid to 5 parts of water. It contains about 10% nitric acid. The specific gravity of concentrated nitric acid is 1.42.
Hydrochloric acid (HCl). Hydrochloric acid is made by dissolving hydrogen chloride gas in water (hydrogen chloride gas is extremely soluble in water). Concentrated hydrochloric acid contains about 38% by weight of hydrogen chloride. Ordinary dilute hydrochloric acid is made by adding 1 part of concentrated hydrochloric acid to 4 parts of water. Such a solution contains from 6% to 8% of hydrogen chloride. The specific gravity of concentrated hydrochloric acid is around 1.20.
Def. Acid anhydride. The oxide of a nonmetal which combines with water to form an acid.
Examples.
1) From the reaction
CO2 + H2O ⇄ H2CO3
we see that CO2 is an acid anhydride of H2CO3.
2) From the reaction
SO3 + H2O ⇄ H2SO4
we see that SO3 is an acid anhydride of H2SO4.
Two general methods for preparing acids
1. Through the reaction of water with the oxide of a metal (i.e. with an acid anhydride).
Examples.
1) We can prepare sulfurous acid by combining water with SO2
SO2 + H2O ⇄ H2SO3
2) We can prepare sulfuric acid by combining water with SO3
SO3 + H2O ⇄ H2SO4
2. Through the reaction of sulfuric acid with a salt. In this general method we heat a salt of the acid we wish to prepare with sulfuric acid.
Examples.
1) To prepare hydrochloric acid we heat sodium chloride and sulfuric acid
NaCl + H2SO4 →NaHSO4 + HCl↑
2) To prepare nitric acid we heat sodium nitrate and sulfuric acid
NaNO3 + H2SO4 →NaHSO4 + HNO3↑
Properties of bases
1. A base contains a metal and one or more hydroxyl (OH) groups.
Examples. NaOH, KOH, Ca(OH)2
The number of OH groups is equal to the valence of the metal. When a base dissociates, the metal of the base becomes the positive ion and the hydroxyl (OH) radical forms the negative ion.
NaOH → Na+ + OH-
KOH → K+ + OH-
Ca(OH)2 → Ca++ + 2 OH-
2. Soluble bases have a bitter taste.
3. A solution of a soluble base is caustic, attacks the skin, and feels slippery.
4. Soluble bases affect indicators, such as litmus paper, causing a color change (insoluble bases generally do not).
5. Bases neutralize acids.
6. Bases react with the oxides of nonmetals forming salts and water.
Example. CO2 + 2 NaOH → NaCO3 + H2O
Naming of bases. The name of a base is formed by adding to the name of the metallic ion the word hydroxide.
Examples. NaOH is sodium hydroxide. Zn(OH)2 is zinc hydroxide. Bi(OH)3 is bismuth hydroxide.
Def. Basic anhydride. The oxide of a metal which combines with water to form an base.
Examples. In the reactions
CaO + H2O → Ca(OH)2
MgO + H2O → Mg(OH)2
CaO and MgO seen to be basic anhydrides.
Three common methods for preparing bases
1. Some active metals react with water to form bases. Such active metals as sodium and potassium react with water with great vigor, liberating hydrogen and forming strong, active bases:
2Na + 2 HOH →2 NaOH + H2↑
2K + 2 HOH →2 KOH + H2↑
2. The oxides of some metals unite with water to form bases (i.e. basic anhydrides unite with water to form bases).
Example. CaO + H2O → Ca(OH)2
3. Insoluble bases may be formed from soluble bases by treating a soluble salt of some metal with a soluble base.
Example. If we add the soluble salt ferric chloride to the soluble base sodium hydroxide we obtain as a precipitate the insoluble base ferric hydroxide.
FeCl3 + 3 NaOH →Fe(OH)3↓+ 3 NaCl
Nearly all dense metals form insoluble bases.
pH scale. The pH of a solution is a measure of the acidity (or alkalinity) of the solution. Neutral solutions have a pH of exactly 7. Numbers less than 7 indicate the presence of an acid and numbers greater than 7 indicate the presence of a base. pH can be measured electrically with a pH meter or with various color change indicators.
The pH of an aqueous solution is the negative log of the activity of the hydrogen ion in the solution.
Normal solutions
Def. Normal solution. A solution containing 1 gram-equivalent weight of solute per liter of solution i.e. a solution with a normality of 1. Also termed a 1 normal or1-N solution.
Examples.
1. A normal solution of HCl would consist of 36.5 g of HCl in a liter of water since the gram-equivalent weight of HCl is 36.5 g.
2. A normal solution of K2SO4 would consist of 87 g of K2SO4 in a liter of water since the gram-equivalent weight of K2SO4 is 87 g (K2SO4 has a formula weight of 174 and a valence of 2, so 174/2 = 87).

● A 2-N solution is one with a normality of 2, a 0.1-N solution is one with a normality of 0.1, and a x-N solution is a solution with a normality of x.
● Normal acid solutions neutralize normal acid solutions milliliter for milliliter. Equal volumes of solutions of equal normality are equivalent.
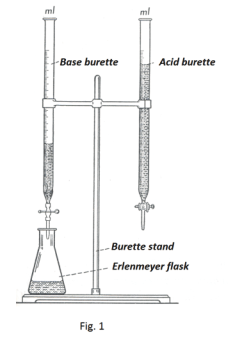
Titration. Suppose a chemist would like to know the percentage of acetic acid in a particular vinegar. To find out he would titrate the vinegar against an acid of known normality using a pair of burettes in a process called titration. See Fig. 1. He proceeds as follows:
1. He fills one of the burettes with the vinegar and the other with 0.1-N base.
2. He draws off 10 ml of vinegar into an Erlenmeyer flask and dilutes it with about 150 ml of water.
3. He adds two drops of a solution of phenolphthalein which is an indicator that is colorless in an acid solution but red in a base solution.
4. He now draws off from the base burette just enough of the 0.1-N base to neutralize the acid in the vinegar, adding it drop by drop until the solution turns a pale pink.
Suppose it takes 80 ml of the 0.1-N base to neutralize the 10 ml of the vinegar. That would mean that 80 ml of base was equivalent to 10 ml of acid and so the acid must be eight times as concentrated as the base. Thus the vinegar must be 0.8-N acetic acid. If 1-N acetic acid contains 60 g of acetic acid per liter, then 0.8-N acetic acid contains 48 g (0.8 × 60) per liter. A liter of 0.8-N acetic acid weighs approximately 1000 g. In 100 g of the sample there is then 4.8 g of acetic acid and the strength is thus 4.8%.
Properties of salts. There are hundreds of salts and their properties vary widely. One of the most important properties of a salt is its solubility.
Solubility of salts in water
1. Common sodium, potassium, and ammonium compounds are soluble in water.
2. Common nitrates, acetates, and chlorates are soluble.
3. Common chlorides are soluble except for silver, mercurous, and lead.
4. Common sulfates are soluble except for calcium, barium, strontium, and lead. (Lead chloride is soluble in hot water.)
5. Common carbonates, phosphates, and silicates are insoluble except sodium, potassium, and ammonium.
6. Common sulfides are insoluble except for calcium, barium, strontium, magnesium, sodium, potassium, and ammonium.
Methods of preparing salts
1. Direct union of the elements.
Example. 2 Na + Cl2 → 2 NaCl
2. Replacement of the hydrogen of an acid by a metal.
Example. 2 Na + 2 HCl →2 NaCl + H2↑
3. Reaction of the oxide of a metal with an acid.
Example. Na2O + 2HCl → 2 NaCl + H2O
4. Reaction of the oxide of a nonmetal with an base.
Example. CO2 + Ca(OH)2 → CaCO3↓ + H2O
5. Neutralize a base with an acid to form a salt.
Example. Sodium hydroxide is added to hydrochloric acid until the solution is neutral.
NaOH + HCl → NaCl + H2O
The solution is evaporated and sodium chloride remains.
Many different salts can be prepared by neutralization.
6. Two salts can be prepared at one time by double replacement.
Example. Add a solution of sodium sulfate to a solution of barium chloride
and two salts are formed — sodium chloride and barium sulfate.
BaCl2 + Na2SO4 →2 NaCl + BaSO4↓
Barium sulfate is insoluble and precipitates out. The water can then be evaporated to obtain crystals of sodium chloride.
Note. It is impossible to separate two salts unless one is soluble and the other is insoluble.
7. Action of an acid on a carbonate to form a salt.
Example. 2HCl + Na2CO3 → 2 NaCl + H2O + CO2↑
Naming of salts. Salt names are formed from the names of their constituent ions.
Examples. Ba(NO3)2 is barium nitrate. FeCl3 is ferric chloride. FeCl2 is ferrous chloride. BaSO4 is barium sulfate.
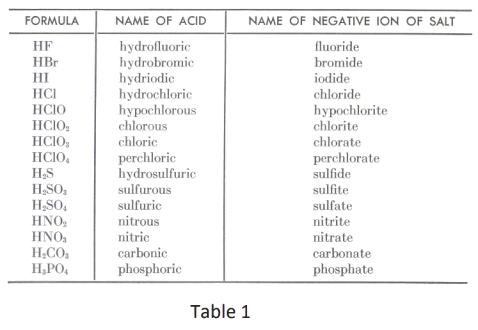
Table 1 gives the names of various acids and the names of the negative ions of the salts derived from the acids.
References
Dull, Brooks, Metcalfe. Modern Chemistry.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life