Website owner: James Miller
Chemical bonds. Electrovalent, covalent and coordinate covalent bonds.

Types of chemical bonds. Atoms bind together to form molecules through three different types of chemical bonds:
1) electrovalent
2) covalent
3) coordinate covalent
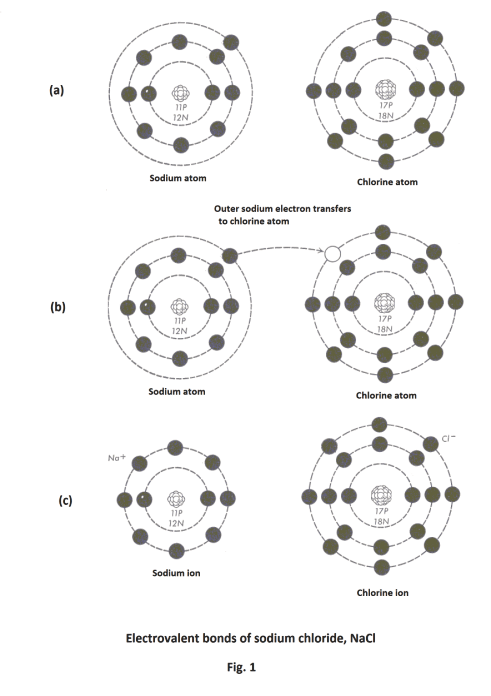
Electrovalent bonds. In forming electrovalent bonds electrons are actually transferred from the outer orbit of one atom to the outer orbit of a second atom. In this process both atoms usually obtain completed outer orbits. For example, in sodium chloride the single electron in the outer orbit of the sodium atom is transferred to the outer orbit of the chlorine atom, completing its outer orbit. This leaves both the sodium and the chloride atoms with completed outer orbits. See Fig. 1. In this process the sodium atom becomes a sodium Na+ ion with a +1 charge and the chlorine atom becomes a chlorine Cl- ion with a -1 charge. These two ions are bound together into a molecule by the force of electrostatic attraction existing between two opposite charges. Note that the process leaves the sodium atom with the electronic structure of the noble gas neon and the chlorine atom with the electronic structure of argon. See Fig. 1 (c).
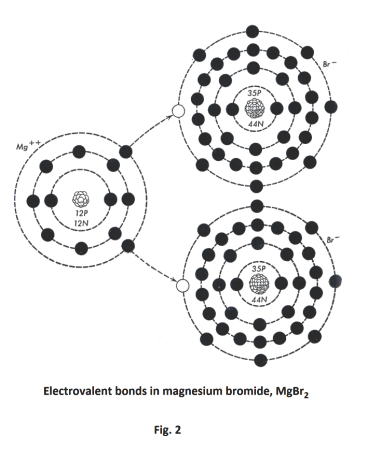
Another example of electrovalent bonding is found in the magnesium bromide MgBr2 molecule. Here one of the two electrons in the outer orbit of magnesium is transferred to one bromine atom and the other is transferred to the other bromine atom. See. Fig. 2. This gives a Mg++ ion and two Br- ions. The ions are bound together as a molecule by the electrostatic forces existing between dissimilar charges.
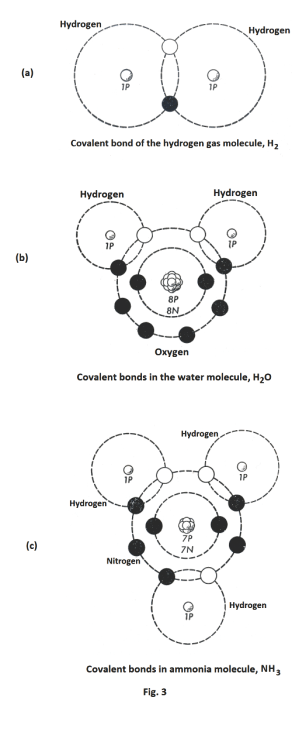
Covalent bonds. In covalent bonds electrons are not transferred from one atom to another as in the electrovalent bond. Instead, two atoms share a pair of electrons with each other. Each of the two atoms contributes one of the electrons of the electron pair. Both electrons in the pair orbit both nuclei. This produces a bond between the two atoms. See Fig. 3 (a) for the covalent bond of hydrogen gas. By this process of sharing pairs of electrons, the atoms involved achieve completed outer orbits which provides them with chemical stability. The bonds of most common gases including hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), and chlorine (Cl2) have covalent bonds. The bonds in water (H2O) and ammonia (NH3) are of the covalent type. See Figs. 3 (b) and 3 (c).
Coordinate covalent bonds. A coordinate covalent bond is like a covalent bond with the only difference being that both electrons in the electron pair come from the same atom.
Syn. Coordinate bond, dative covalent bond, dipolar bond.
Note. Definitions differ. In some definitions the covalent bond is defined so as to include the coordinate covalent case. A coordinate covalent bond is then simply a special case of a covalent bond where both electrons come from the same atom.
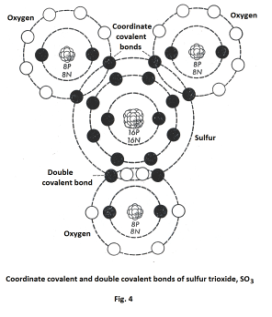
The sulfur trioxide molecule has both a coordinate covalent bond and a double covalent bond. See Fig. 4.
References
Dull, Brooks, Metcalfe. Modern Chemistry.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life