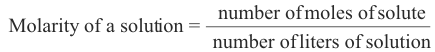
Website owner: James Miller
Ways of expressing concentrations of solutions. Solute, solvent. Molarity, Formality, Normality, Molality, Mole fraction. Dilution problems.
Def. Solution. A homogeneous mixture formed by dissolving one or more substances, whether solid, liquid, or gaseous, in another substance.
Solute and solvent. In a solution the dissolved substance is called the solute and the substance in which the solute is dissolved is called the solvent. In a solution consisting of the mixture of two liquids, the liquid present in the larger amount is generally regarded as the solvent.
Ways of expressing concentrations of solutions in physical units:
1. By weight of solute per unit volume of solution.
Example. 10 g of NaCl per liter of solution.
2. By percentage composition i.e. the number of grams of solute per 100 grams of solution.
Example. A 10% NaCl solution contains 10 grams of NaCl per 100 grams of solution. Ten grams of NaCl are mixed with 90 grams or milliliters of water to form 100 grams of solution.
3. By weight of solute per weight of solvent.
Example. 6 g of NaCl per 100 g of water.
Ways of expressing concentrations of solutions in chemical units:
1. Molarity. The molarity of a solution is the number of moles of the solute contained in one liter of the solution.
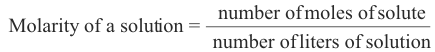
2. Formality. The formality of a solution is the number of gram-formula weights of the solute contained in one liter of the solution.
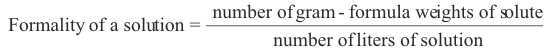
3. Normality. The normality of a solution is the number of gram-equivalent weights of the solute contained in one liter of the solution.
![]()
![]()
where a milligram-equivalent is 1/1000 of a gram-equivalent weight.
4. Molality. The molality of a solution is the number of moles of the solute per kilogram of solvent.
Syn. Weight molarity
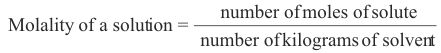
5. Mole fraction. The mole fraction of one component in a solution is defined as the number of moles of that component divided by the total number of moles of all components in the solution. The sum of the mole fractions of all components of a solution is 1. In a two-component solution consisting of solute plus solvent, the mole fraction of the solute is
![]()
and the mole fraction of solvent is
![]()
In general,
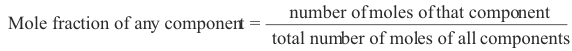
Dilution problems. Volume-referenced measures of concentration are those in which concentration is expressed as x units of solute per unit volume. For example, moles per liter (molarity), gram-formula weights per liter (formality), gram-equivalent weights per liter (normality), and grams per liter are volume-referenced measures.
If concentration is expressed as x units of solute per unit volume, then the total amount of solute in solution is given by
1) Amount of solute = volume × concentration
Example. A NaCl solution has a concentration of 7 g per liter. Then the amount of solute in 5 liters is given by
Amount of solute = 5×7 = 35 g
When a solution is diluted, the volume is increased and the concentration is decreased, but the total amount of solute in the solution remains the same. Thus if v1 and c1 are the volume and concentration of the solution before dilution and v2 and c2 are the volume and concentration of the solution after dilution, then
2) v1 × c1 = v2 × c2
In a dilution problem, if any three of these variables are known, the other can be calculated.
Problem. A solution of AgNO3 has a concentration of 40 mg per ml. How much must this solution be diluted to yield a concentration of 10 g per ml?
Solution. Using 2) above
v1 × c1 = v2 × c2
1 ml × 40 mg/ml = v2 × 10 mg/ml
v2 = 40/10 = 4 ml
Thus each ml of the given solution must be diluted to a volume of 4 ml.
References
Schaum, Beckman, Rosenberg. College Chemistry. (Schaum)
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life