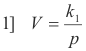
Website owner: James Miller
Proof. Given Vp = k1 at constant temperature and V = k2T at constant pressure, we wish to prove that Vp = kT.
Because Boyle’s law is applicable only at constant temperature and the Charles-Gay-Lussac is only applicable at constant pressure, it is not immediately clear that simple combination of the two laws is justified. However, Boyle’s law can be written as
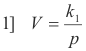
and so it is clear that the volume is a function of the pressure and the absolute temperature i.e.
2] V = f(p, T)
By partial differentiation we obtain
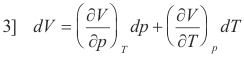
Taking the derivative of 1] with respect to p we get

Taking the derivative of V = k2T with respect to T
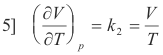
Substituting 4] and 5] into 3] we obtain
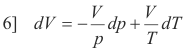
or
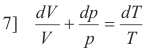
Integrating
8] ln V + ln p = ln T + ln k (a constant)
Taking antilogarithms we get
9] pV = kT